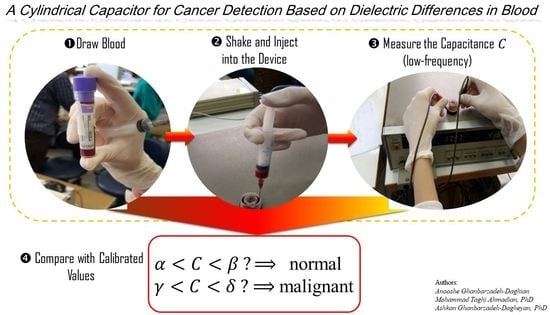
Quick, Single-Frequency Dielectric Characterization of Blood Samples of Pediatric Cancer Patients by a Cylindrical Capacitor: Pilot Study
Abstract
1. Introduction
2. Materials and Methods
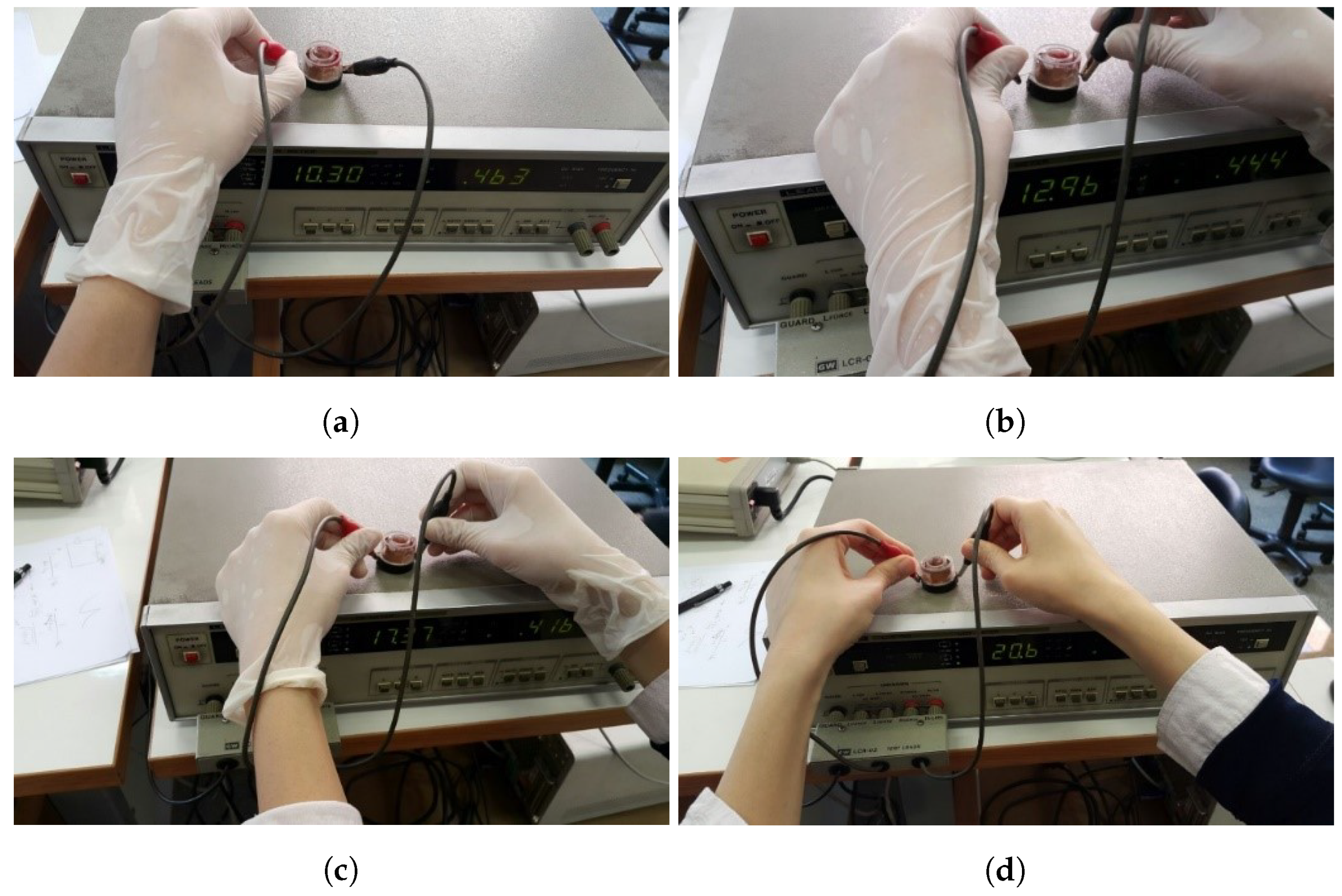
2.1. Setup
2.2. Experiment and Data Collection
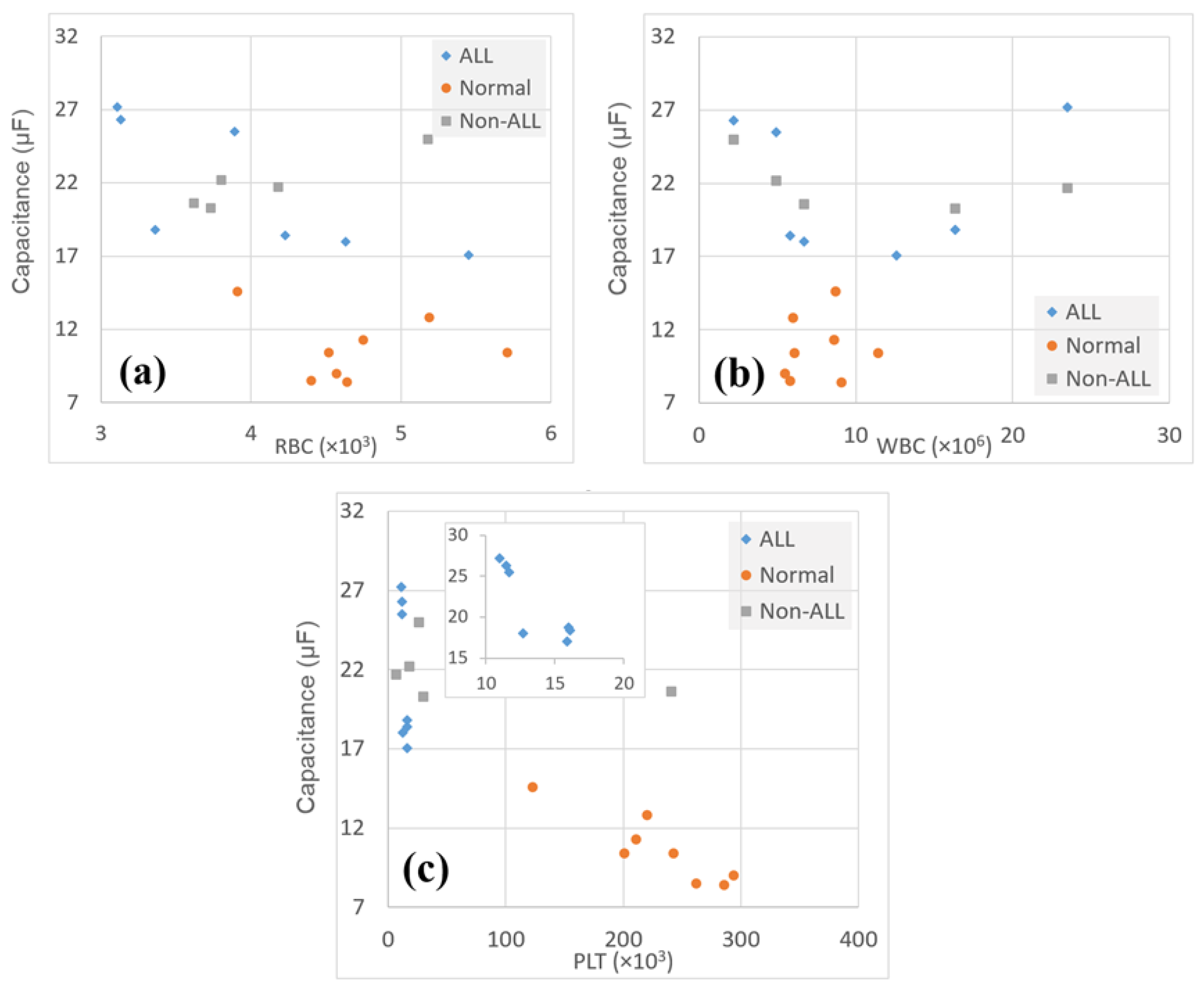
3. Results
4. Discussion
4.1. Potential Mechanisms behind the Study Observations
4.2. Study Limitations
- It is well known that pediatric cancer research, due to nonprofitablity, is, sadly, a greatly underfunded (almost neglected) field of research. A look at many original studies on minor cancer patients reveals that the sample sizes are generally small, as in this study. For example, the authors in [73,74,75], respectively, had 17, 20, and 28 subjects in their study. Further, in a previous paper by the two authors of the study at hand, only 11 children remained until the end of the study for the experimental and control group, combined [76,77]. This could be due to a lack of adequate funding to conduct large scale studies on children suffering from cancer, as well as the difficulty in recruiting these children in the study.
- In this research, due to the study design and taking into account the effect of common drugs for cancer therapy, it was necessary to find recently diagnosed cancer patients, in a specified age range, who had not yet gone under any treatment (chemotherapy, radiotherapy, etc.). Besides, among the eligible subjects, it was still required to have the consent of their parents for obtaining blood samples. Considering their situation and the fact that frequent blood draw and injections already cause discomfort for pediatric patients, only one sample was obtained from each eligible patient.
- This was a proof-of-concept study, aimed at testing a hypothesis, with limited resources, for further, more thorough investigations in the future. Well conducted small scale studies, particularly the ones that do not deal with developing new medical drugs, have the distinct benefit of reporting a new observation or phenomenon. Novel ideas can compensate for the justified shortage of data, as the case reported in [77].
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALL | Acute lymphocytic leukemia |
| CBC | Complete blood count |
| PLT | Platelets |
| RBC | Red blood cell |
| WBC | White blood cell |
References
- Xu, Y.; Xie, X.; Duan, Y.; Wang, L.; Cheng, Z.; Cheng, J. A review of impedance measurements of whole cells. Biosens. Bioelectron. 2016, 77, 824–836. [Google Scholar] [CrossRef]
- Connelly, J.; Buckler, M. The continuous measurement of resistivity and permittivity of human blood plasma during coagulation. Med Biol. Eng. 1975, 13, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.X.; Jacobson, B.; Ribbe, T. Triple-frequency method for measuring blood impedance. Physiol. Meas. 1993, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Meijer, G.C.; Pop, G. A novel model of blood impedance for indirect viscosity measurement. Proc. Electron., Sozopol, Bulgaria, 22–24 September 2004. Available online: https://ecad.tu-sofia.bg/et/2004/Papers/Electronic%20Medical%20Equipment/Paper-Zu-yao.pdf (accessed on 13 December 2019).
- Zhbanov, A.; Yang, S. Electrochemical impedance spectroscopy of blood for sensitive detection of blood hematocrit, sedimentation and dielectric properties. Anal. Methods 2017, 9, 3302–3313. [Google Scholar] [CrossRef]
- Pradhan, R.; Mitra, A.; Das, S. Quantitative evaluation of blood glucose concentration using impedance sensing devices. J. Electr. Bioimpedance 2019, 4, 73–77. [Google Scholar] [CrossRef]
- Dai, T.; Adler, A. Blood impedance characterization from pulsatile measurements. In Proceedings of the 2006 Canadian Conference on Electrical and Computer Engineering, Ottawa, ON, Canada, 7–10 May 2006; pp. 983–986. [Google Scholar]
- Wang, M.H.; Jang, L.S. A systematic investigation into the electrical properties of single HeLa cells via impedance measurements and COMSOL simulations. Biosens. Bioelectron. 2009, 24, 2830–2835. [Google Scholar] [CrossRef]
- Nwankire, C.E.; Venkatanarayanan, A.; Glennon, T.; Keyes, T.E.; Forster, R.J.; Ducrée, J. Label-free impedance detection of cancer cells from whole blood on an integrated centrifugal microfluidic platform. Biosens. Bioelectron. 2015, 68, 382–389. [Google Scholar] [CrossRef]
- Asami, K. Dielectric properties of dipicrylamine-doped erythrocytes, cultured cells and lipid vesicles. Bioelectrochemistry 2013, 92, 14–21. [Google Scholar] [CrossRef][Green Version]
- Di Biasio, A.; Cametti, C. Effect of the shape of human erythrocytes on the evaluation of the passive electrical properties of the cell membrane. Bioelectrochemistry 2005, 65, 163–169. [Google Scholar] [CrossRef]
- Yoon, G. Dielectric properties of glucose in bulk aqueous solutions: Influence of electrode polarization and modeling. Biosens. Bioelectron. 2011, 26, 2347–2353. [Google Scholar] [CrossRef]
- Beving, H.; Eriksson, L.; Davey, C.; Kell, D. Dielectric properties of human blood and erythrocytes at radio frequencies (0.2–10 MHz); dependence on cell volume fraction and medium composition. Eur. Biophys. J. 1994, 23, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Zhao, K.; Asami, K. Effects of copper on dielectric properties of E. coli cells. Colloids Surf. B Biointerfaces 2007, 58, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Markx, G.H. Dielectric measurement of cell death. Enzym. Microb. Technol. 2008, 43, 463–470. [Google Scholar] [CrossRef]
- Baskurt, O.; Uyuklu, M.; Meiselman, H. Simultaneous monitoring of electrical conductance and light transmittance during red blood cell aggregation. Biorheology 2009, 46, 239–249. [Google Scholar] [CrossRef]
- Jung, J.M.; Lee, D.H.; Kim, K.T.; Cho, Y.I. Determination of hematocrit using on-line conductance cell. Int. J. Heat Mass Transf. 2012, 55, 1836–1843. [Google Scholar] [CrossRef]
- Jaspard, F.; Nadi, M. Dielectric properties of blood: An investigation of temperature dependence. Physiol. Meas. 2002, 23, 547. [Google Scholar] [CrossRef]
- Sun, L.; Collins, C.; Schiano, J.L.; Smith, M.; Smith, N. Adaptive real-time closed-loop temperature control for ultrasound hyperthermia using magnetic resonance thermometry. Concepts Magn. Reson. Part B Magn. Reson. Eng. Educ. J. 2005, 27, 51–63. [Google Scholar] [CrossRef]
- Sigman, E.; Kolin, A.; Katz, L.; Jochim, K. Effect of motion on the electrical conductivity of the blood. Am. J. Physiol. Leg. Content 1937, 118, 708–719. [Google Scholar] [CrossRef]
- Schwan, H. Electrical properties of blood and its constitutents: Alternating current spectroscopy. Blut Zeitschrift für die Gesamte Blutforschung 1983, 46, 185–197. [Google Scholar] [CrossRef]
- Grimnes, S.; Martinsen, ØG. Alpha-dispersion in human tissue. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2010; Volume 224, p. 012073. [Google Scholar]
- Wolf, M.; Gulich, R.; Lunkenheimer, P.; Loidl, A. Broadband dielectric spectroscopy on human blood. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 727–740. [Google Scholar] [CrossRef]
- Farsaci, F.; Tellone, E.; Cavallaro, M.; Russo, A.; Ficarra, S. Low frequency dielectric characteristics of human blood: A non-equilibrium thermodynamic approach. J. Mol. Liq. 2013, 188, 113–119. [Google Scholar] [CrossRef]
- Zhuang, J.; Kolb, J.F. Time domain dielectric spectroscopy of nanosecond pulsed electric field induced changes in dielectric properties of pig whole blood. Bioelectrochemistry 2015, 103, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, F.G.; Texter, E.C.; Wood, L.A.; Ballard, W.C.; Horan, F.E.; Wright, I.S.; Frey, C.; Starr, D. The electrical conductivity of blood: I. Relationship to erythrocyte concentration. Blood 1950, 5, 1017–1035. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.S.A.; Lei, K.F.; Hwang, Y.S.; Zhang, J.H.; Lei, T.C. Impedimetric detection of whole blood concentration for early detection of intraocular hemorrhage. Microelectron. Eng. 2014, 129, 70–76. [Google Scholar] [CrossRef]
- Abdalla, S.; Al-Ameer, S.; Al-Magaishi, S. Electrical properties with relaxation through human blood. Biomicrofluidics 2010, 4, 034101. [Google Scholar] [CrossRef]
- Chen, X.; Lv, X.; Wang, H. Lung carcinoma recognition by blood dielectric spectroscopy. Bio-Med. Mater. Eng. 2015, 26, S895–S901. [Google Scholar] [CrossRef]
- Batyuk, L.; Kizilova, N. Dielectric Properties of Red Blood Cells for Cancer Diagnostics and Treatment. Acta Sci. Cancer Biol. 2018, 2, 10. Available online: https://www.actascientific.com/ASCB/pdf/ASCB-02-0079.pdf (accessed on 13 December 2019).
- Moqadam, S.M.; Grewal, P.K.; Haeri, Z.; Ingledew, P.A.; Kohli, K.; Golnaraghi, F. Cancer detection based on electrical impedance spectroscopy: A clinical study. J. Electr. Bioimpedance 2018, 9, 17–23. [Google Scholar] [CrossRef]
- Huerta-Nuñez, L.; Gutierrez-Iglesias, G.; Martinez-Cuazitl, A.; Mata-Miranda, M.; Alvarez-Jiménez, V.; Sánchez-Monroy, V.; Golberg, A.; González-Díaz, C. A biosensor capable of identifying low quantities of breast cancer cells by electrical impedance spectroscopy. Sci. Rep. 2019, 9, 6419. [Google Scholar] [CrossRef]
- Bueno Barrachina, J.M.; Cañas Peñuelas, C.S.; Catalán Izquierdo, S. FEM edge effect and capacitance evaluation on cylindrical capacitors. J. Energy Power Eng. 2012, 6, 2063–2069. [Google Scholar]
- Rusiniak, L. Electric properties of water. New experimental data in the 5 Hz–13 MHz frequency range. Acta Geophys. Pol. 2004, 52, 63–76. [Google Scholar]
- Parikh, R.; Mathai, A.; Parikh, S.; Sekhar, G.C.; Thomas, R. Understanding and using sensitivity, specificity and predictive values. Indian J. Ophthalmol. 2008, 56, 45. [Google Scholar] [CrossRef] [PubMed]
- Gligoroska, J.P.; Gontarev, S.; Dejanova, B.; Todorovska, L.; Stojmanova, D.S.; Manchevska, S. Red Blood Cell Variables in Children and Adolescents regarding the Age and Sex. Iran. J. Public Health 2019, 48, 704. [Google Scholar]
- Amin, M.; Dey, P.P.; Badkoobehi, H. A complete electrical equivalent circuit model for biological cell. In Proceedings of the 7th WSEAS International Conference on Applied Computer and Applied Computational Science, Hangzhou, China, 6–8 April 2008; pp. 343–348. [Google Scholar]
- Sepehri, A. A mathematical model for DNA. Int. J. Geometr. Methods Mod. Phys. 2017, 14, 1750152. [Google Scholar] [CrossRef]
- Petrov, A.G. Flexoelectricity of model and living membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2002, 1561, 1–25. [Google Scholar] [CrossRef]
- Adey, W. Ionic Nonequilibrium Phenomena in Tissue Interactions with Electromagnetic Fields; ACS Publications: Washington, DC, USA, 1981. [Google Scholar]
- Alqabandi, J.A.; Abdel-Motal, U.M.; Youcef-Toumi, K. Extracting cancer cell line electrochemical parameters at the single cell level using a microfabricated device. Biotechnol. J. 2009, 4, 216–223. [Google Scholar] [CrossRef]
- Ambrose, E.; James, A.; Lowick, J. Differences between the electrical charge carried by normal and homologous tumour cells. Nature 1956, 177, 576. [Google Scholar] [CrossRef]
- Becker, R. The electrical control of growth processes. Med. Times 1967, 95, 657–669. [Google Scholar]
- Hammerick, K.E.; Longaker, M.T.; Prinz, F.B. In vitro effects of direct current electric fields on adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 2010, 397, 12–17. [Google Scholar] [CrossRef]
- Popp, F.A. Cancer growth and its inhibition in terms of coherence. Electromagn. Biol. Med. 2009, 28, 53–60. [Google Scholar] [CrossRef]
- Becker, R.O. The basic biological data transmission and control system influenced by electrical forces. Ann. N. Y. Acad. Sci. 1974, 238, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Haltiwanger, S. Clinical Use of Mineral Transporters and Their Effects on Cell Membrane Capacitance: Second International Congress of BioEnergetic Medicine; Institute of Quantum and Molecular Medicine: Melbourne, FL, USA, 1998. [Google Scholar]
- Blackiston, D.; Adams, D.S.; Lemire, J.M.; Lobikin, M.; Levin, M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis. Models Mech. 2011, 4, 67–85. [Google Scholar] [CrossRef] [PubMed]
- Brewer, A.K.; Passwater, R. Physics of the cell membrane. Mechanisms involved in cancer. Am Lab 1976, 10, 37–45. [Google Scholar]
- Ling, G. The association-induction hypothesis. A theoretical foundation provided for the possible beneficial effects of a low Na, high K diet and other similar regimens in the treatment of patients suffering from debilitating illnesses. Agressol. Rev. Int. Phys.-Biol. Pharmacol. Appl. Eff. L’agression 1983, 24, 293. [Google Scholar]
- Cheng, N.; Van Hoof, H.; Bockx, E.; Hoogmartens, M.J.; Mulier, J.C.; De Dijcker, F.J.; Sansen, W.M.; De Loecker, W. The effects of electric currents on ATP generation, protein synthesis, and membrane transport of rat skin. Clin. Orthop. Relat. Res. 1982, 171, 264–272. [Google Scholar] [CrossRef]
- Dhar, G.; Sen, S.; Chaudhuri, G. Acid gradient across plasma membrane can drive phosphate bond synthesis in cancer cells: Acidic tumor milieu as a potential energy source. PLoS ONE 2015, 10, e0124070. [Google Scholar] [CrossRef]
- Hsu, P.P.; Sabatini, D.M. Cancer cell metabolism: Warburg and beyond. Cell 2008, 134, 703–707. [Google Scholar] [CrossRef]
- Marin-Hernandez, A.; Gallardo-Perez, J.C.; Ralph, S.J.; Rodriguez-Enriquez, S.; Moreno-Sanchez, R. HIF-1α modulates energy metabolism in cancer cells by inducing over-expression of specific glycolytic isoforms. Mini Rev. Med. Chem. 2009, 9, 1084–1101. [Google Scholar] [CrossRef]
- Annibaldi, A.; Widmann, C. Glucose metabolism in cancer cells. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 466–470. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Ojugo, A.S.; McSheehy, P.M.; McIntyre, D.J.; McCoy, C.; Stubbs, M.; Leach, M.O.; Judson, I.R.; Griffiths, J.R. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: A comparison of exogenous 19F and 31P probes. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. In Vivo 1999, 12, 495–504. [Google Scholar]
- Ridgway, P.F.; Ziprin, P.; Peck, D.H.; Darzi, A.W. Hypoxia increases reepithelialization via an αvβ6-dependent pathway. Wound Repair Regen. 2005, 13, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Ilc, K.; Laferrière, J.; Trottier, E.; Dayan, F.; Mazure, N.M.; Brahimi-Horn, M.C.; Pouysségur, J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009, 69, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, C.L.; Weigel, K.J.; Schafer, Z.T. Cancer cell survival during detachment from the ECM: Multiple barriers to tumour progression. Nature Reviews Cancer 2014, 14, 632–641. [Google Scholar] [CrossRef]
- Nukpezah, T.; Golemis, E.A. The Extracellular matrix and ciliary signaling. Curr. Opin. Cell Biol. 2012, 24, 652–661. [Google Scholar] [CrossRef]
- Zhuo, Y.; Bellis, S. Emerging role of α2, 6-sialic acid as a negative regulator of galectin binding and function. J. Biol. Chem. 2011, 286, 5935–5941. [Google Scholar] [CrossRef]
- Board, M.; Colquhoun, A.; Newsholme, E.A. High Km glucose-phosphorylating (glucokinase) activities in a range of tumor cell lines and inhibition of rates of tumor growth by the specific enzyme inhibitor mannoheptulose. Cancer Res. 1995, 55, 3278–3285. [Google Scholar]
- Blad, B.; Baldetorp, B. Impedance spectra of tumour tissue in comparison with normal tissue; a possible clinical application for electrical impedance tomography. Physiol. Meas. 1996, 17, A105. [Google Scholar] [CrossRef]
- Halter, R.J.; Schned, A.; Heaney, J.; Hartov, A.; Schutz, S.; Paulsen, K.D. Electrical impedance spectroscopy of benign and malignant prostatic tissues. J. Urol. 2008, 179, 1580–1586. [Google Scholar] [CrossRef]
- Foster, K.; Schepps, J. Dielectric properties of tumor and normal tissues at radio through microwave frequencies. J. Microw. Power 1981, 16, 107–119. [Google Scholar] [CrossRef]
- Belmonte, R.; Tejero, M.; Ferrer, M.; Muniesa, J.M.; Duarte, E.; Cunillera, O.; Escalada, F. Efficacy of low-frequency low-intensity electrotherapy in the treatment of breast cancer-related lymphoedema: A cross-over randomized trial. Clin. Rehabil. 2012, 26, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen, A.M.; DeWitt, M.R.; Rylander, M.N. Nanoparticle enhanced optical imaging and phototherapy of cancer. J. Biomed. Nanotechnol. 2014, 10, 1677–1712. [Google Scholar] [CrossRef] [PubMed]
- Olaku, O.; White, J.D. Herbal therapy use by cancer patients: A literature review on case reports. Eur. J. Cancer 2011, 47, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421. [Google Scholar] [PubMed]
- Tong, J.; Liu, R.; Zhao, L.; Kong, W.; Tang, J. Inhibiting human breast cancer cells (mcf-7) with alternating micro-current at intermediate frequency (acif) in vitro and in vivo. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Beijing, China, 26–31 May 2012; pp. 1596–1599. [Google Scholar]
- Ulgen, Y.; Sezdi, M. Electrical parameters of human blood. In Proceedings of the 20th Annual International Conference of the IEEE Engineering in Medicine and Biology Society. Vol.20 Biomedical Engineering Towards the Year 2000 and Beyond (Cat. No.98CH36286), Hong Kong, China, 1 November 1998; Volume 6, pp. 2983–2986. [Google Scholar]
- Barrera, M.; Chung, J.Y.; Greenberg, M.; Fleming, C. Preliminary investigation of a group intervention for siblings of pediatric cancer patients. Child. Health Care 2002, 31, 131–142. [Google Scholar] [CrossRef]
- Hinds, P.S.; Drew, D.; Oakes, L.L.; Fouladi, M.; Spunt, S.L.; Church, C.; Furman, W.L. End-of-life care preferences of pediatric patients with cancer. J. Clin. Oncol. 2005, 23, 9146–9154. [Google Scholar] [CrossRef]
- Chow, A.Y.; Chin, C.; Dahl, G.; Rosenthal, D.N. Anthracyclines cause endothelial injury in pediatric cancer patients: A pilot study. J. Clin. Oncol. 2006, 24, 925–928. [Google Scholar] [CrossRef]
- Alemi, M.; Meghdari, A.; Ghanbarzadeh, A.; Moghadam, L.J.; Ghanbarzadeh, A. Impact of a social humanoid robot as a therapy assistant in children cancer treatment. In Proceedings of the International Conference on Social Robotics, Sydney, NSW, Australia, 27–29 October 2014; pp. 11–22. [Google Scholar]
- Alemi, M.; Ghanbarzadeh, A.; Meghdari, A.; Moghadam, L.J. Clinical application of a humanoid robot in pediatric cancer interventions. Int. J. Soc. Robot. 2016, 8, 743–759. [Google Scholar] [CrossRef]

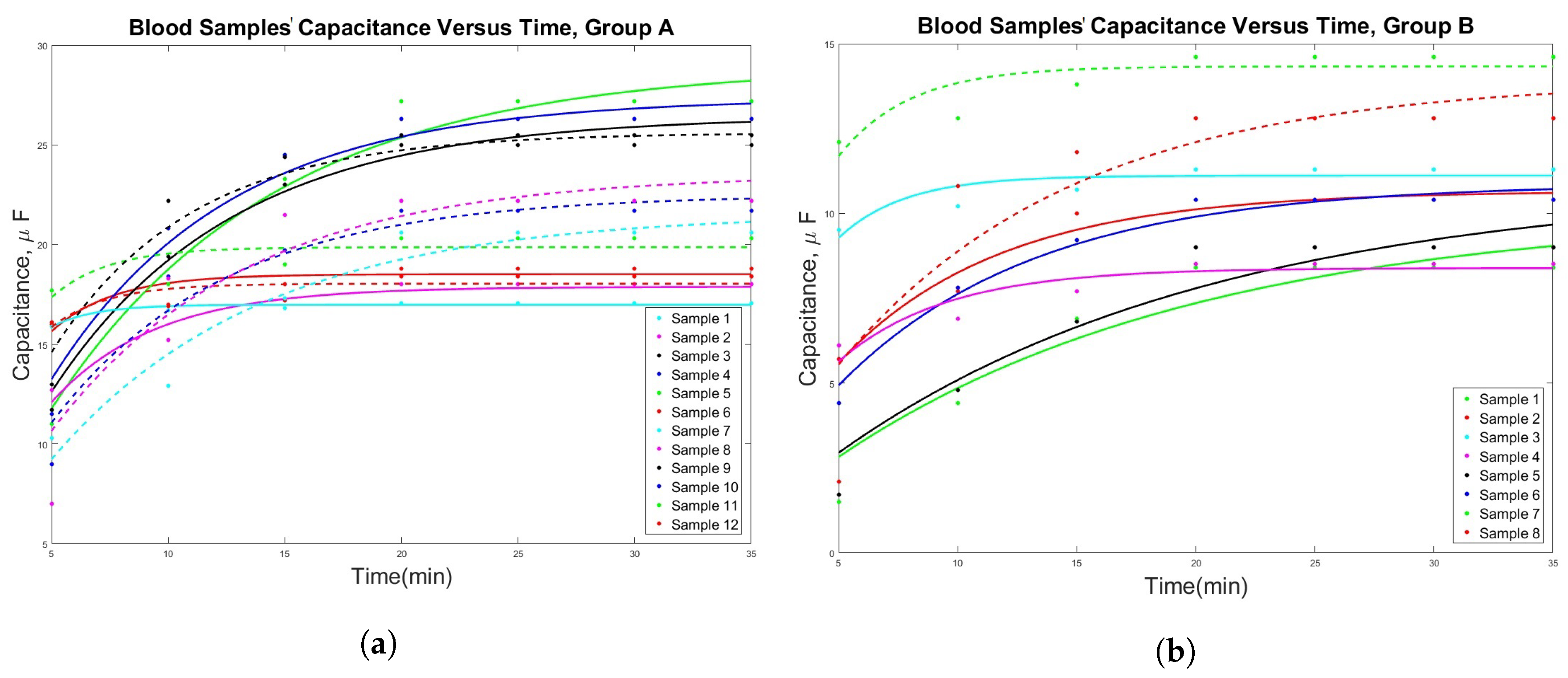
| # | Sex | RBC (L) | WBC (L) | PLTs (L) | C (F) at | C (F) at min | C (F) at min | C (F) at min | () |
|---|---|---|---|---|---|---|---|---|---|
| A | |||||||||
| 1 | F | 3.36 | 16.35 | 30 | 16 | 17 | 18 | 18.8 | 11.4 |
| 2 | M | 3.11 | 23.5 | 7 | 11 | 18 | 23.3 | 27.2 | 16.4 |
| 3 | M | 3.13 | 2.2 | 26 | 11.5 | 20.8 | 24.5 | 26.3 | 15.9 |
| 4 | F | 3.89 | 4.9 | 18 | 11.7 | 19.4 | 23 | 25.5 | 15.4 |
| 5 | F | 3.73 | 5.9 | 9 | 17.7 | 18.3 | 19 | 20.3 | 12.3 |
| 6 | M | 3.8 | 31.2 | 49 | 7 | 18.3 | 21.5 | 22.2 | 13.4 |
| 7 | M | 3.62 | 3.9 | 14 | 10.3 | 12.9 | 17.3 | 20.6 | 12.4 |
| 8 | F | 4.63 | 6.7 | 241 | 12.7 | 15.2 | 16.8 | 18 | 10.9 |
| 9 | F | 5.45 | 12.6 | 183 | 15.9 | 16.7 | 16.8 | 17.1 | 10.3 |
| 10 | M | 4.23 | 5.8 | 285 | 16.1 | 16.9 | 17.2 | 18.4 | 11.1 |
| 11 | M | 4.18 | 8.2 | 166 | 9 | 18.4 | 19.7 | 21.7 | 13.1 |
| 12 | F | 5.18 | 6.2 | 164 | 13 | 22.2 | 24.4 | 25 | 15.1 |
| B | |||||||||
| 13 | F | 4.52 | 6.1 | 201 | 5.7 | 7.7 | 10 | 10.4 | 6.3 |
| 14 | M | 4.64 | 9.1 | 286 | 1.5 | 4.4 | 6.9 | 8.4 | 5.1 |
| 15 | M | 5.71 | 11.4 | 243 | 4.4 | 7.8 | 9.2 | 10.4 | 6.3 |
| 16 | M | 4.57 | 5.5 | 294 | 1.7 | 4.8 | 6.8 | 9 | 5.4 |
| 17 | F | 4.4 | 5.8 | 262 | 6.1 | 6.9 | 7.7 | 8.5 | 5.1 |
| 18 | F | 4.75 | 8.6 | 211 | 9.5 | 10.2 | 10.7 | 11.3 | 6.8 |
| 19 | M | 5.19 | 6 | 220 | 2.1 | 10.8 | 11.8 | 12.8 | 7.7 |
| 20 | M | 3.91 | 8.7 | 123 | 12.1 | 12.8 | 13.8 | 14.6 | 8.8 |
| Corresponding | TP | FN | TN | FP | SE | SP | |
|---|---|---|---|---|---|---|---|
| 15–17 | 9-08–10.28 | 12 | 0 | 8 | 0 | 100% | 100% |
| 18 | 10.9 | 11 | 1 | 8 | 0 | 92% | 100% |
| 20 | 12.09 | 8 | 4 | 8 | 0 | 67% | 100% |
| 22 | 13.30 | 5 | 7 | 8 | 0 | 42% | 100% |
| 14 | 8.46 | 12 | 0 | 7 | 1 | 100% | 88% |
| 12 | 7.26 | 12 | 0 | 6 | 2 | 100% | 75% |
| 10 | 6.04 | 12 | 0 | 3 | 5 | 100% | 38% |
| Parameter | Cancerous Samples | ALL Samples | Normal Samples |
|---|---|---|---|
| RBCs | 4.026 ± 0.075 | 3.520 ± 0.320 | 4.711 ± 0.538 |
| Correlation with Capacitance | −0.44 | −0.51 | −0.15 |
| WBCs | 10.621 ± 8.848 | 12.564 ± 11.305 | 7.650 ± 2.116 |
| Correlation with Capacitance | −0.072 | −0.073 | −0.15 |
| PLTs | 99.333 ± 101.564 | 21.857 ± 16.622 | 230 ± 55.001 |
| Correlation with Capacitance | −0.53 | −0.77 | −0.90 |
| Capacitance | 21.756 ± 3.497 | 22.986 ± 3.319 | 10.675 ± 2.177 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghanbarzadeh-Daghian, A.; Ahmadian, M.T.; Ghanbarzadeh-Dagheyan, A. Quick, Single-Frequency Dielectric Characterization of Blood Samples of Pediatric Cancer Patients by a Cylindrical Capacitor: Pilot Study. Electronics 2020, 9, 95. https://doi.org/10.3390/electronics9010095
Ghanbarzadeh-Daghian A, Ahmadian MT, Ghanbarzadeh-Dagheyan A. Quick, Single-Frequency Dielectric Characterization of Blood Samples of Pediatric Cancer Patients by a Cylindrical Capacitor: Pilot Study. Electronics. 2020; 9(1):95. https://doi.org/10.3390/electronics9010095
Chicago/Turabian StyleGhanbarzadeh-Daghian, Anooshe, Mohammad Taghi Ahmadian, and Ashkan Ghanbarzadeh-Dagheyan. 2020. "Quick, Single-Frequency Dielectric Characterization of Blood Samples of Pediatric Cancer Patients by a Cylindrical Capacitor: Pilot Study" Electronics 9, no. 1: 95. https://doi.org/10.3390/electronics9010095
APA StyleGhanbarzadeh-Daghian, A., Ahmadian, M. T., & Ghanbarzadeh-Dagheyan, A. (2020). Quick, Single-Frequency Dielectric Characterization of Blood Samples of Pediatric Cancer Patients by a Cylindrical Capacitor: Pilot Study. Electronics, 9(1), 95. https://doi.org/10.3390/electronics9010095